News
New tools to increase the reliability of in vivo phage biopanning reported in Nucleic Acids Research
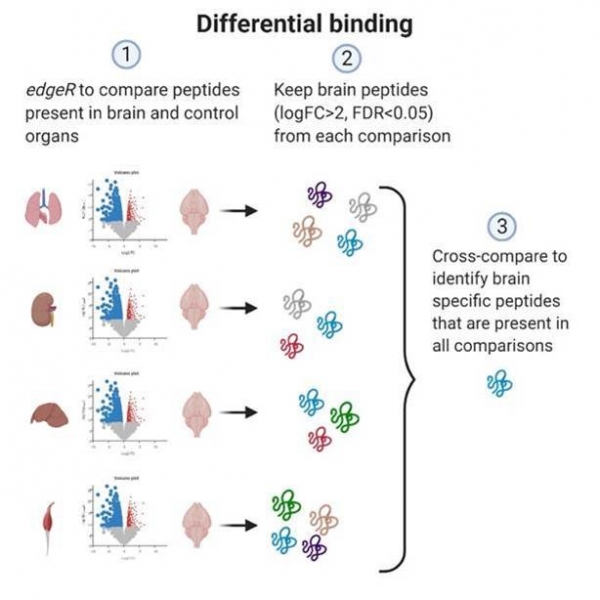
In vivo phage display is widely used for the identification of organ- or disease-specific homing peptides. In our study (lead authors PhD students Karlis Pleiko and Kristina Põšnograjeva), we developed new tools to streamline in vivo biopanning screens. Our approach allows differential mapping of homing ability and organ-selectivity of peptide-displaying phages during in vivo selection based solely on the high throughput sequencing data. This study will accelerate mapping of vascular heterogeneity in vivo and, ultimately, catalyze progress toward development of affinity-guided smart drugs and contrast agents.
“In vivo phage display: identification of organ-specific peptides using deep sequencing and differential profiling across tissues”, Pleiko K, Põšnograjeva K, Haugas M, Paiste P, Tobi A, Kurm K, Riekstina U, Teesalu T; Nucleic Acids Research, (2021 Jan 14; https://doi.org/10.1093/nar/gkaa1279); “;
https://academic.oup.com/nar/advance-article/doi/10.1093/nar/gkaa1279/6097687
Congratulations to Prakash Lingasamy for successfully defending his PhD thesis!
Prakash Lingasamy successfully defended his Ph.D. thesis " Development of multitargeted tumor penetrating peptides" on January 29th, 2021. Opponent of the thesis was professor Angelo Corti from Università Vita-Salute San Raffaele (Milan, Italy). Compared to conventional monospecific tumor homing peptides, multispecific peptides identified in the thesis provide a better accumulation of therapeutic and diagnostic nanoparticles in solid tumors. These preclinical results warrant downstream clinical studies on development of peptide-guided drugs and imaging agents. We wish Prakash good luck in his future career!
https://dspace.ut.ee/handle/10062/70738
https://dspace.ut.ee/bitstream/handle/10062/70738/lingasamy_prakash.pdf
Two back-to-back collaborative studies published in Science on Neuropilin as a novel coronavirus receptor
Our lab was involved in two studies by international research teams that show that new coronavirus uses NRP-1 as a cellular internalization receptor.
The research stems from our earlier work on C-end Rule (CendR) peptides. CendR peptides act as position-dependent cellular switches: when present at C-terminus of any protein, they bind to Neuropilins to trigger cellular uptake and tissue penetration. The SARS-CoV-2 virus uses a protein called Spike to bind and enter host cells. New research shows that proteolytic processing of Spike protein by cellular enzyme furin results in activation of cryptic CendR peptide to trigger cellular uptake and tissue penetration of the CoV2 particles.
This discovery opens a new path for development of coronavirus drugs. Intriguingly, the CendR sequences are present on the surface of other pathogenic viruses, such as Ebola and HIV-1.
Our laboratory provided key reagents related to CendR pathway (antibodies, recombinant proteins, CendR nanoparticles) to the virology labs in Finland, UK, and Germany that coordinated the collaboration (PIs: Drs. Giuseppe Balistreri, Yohei Yamauchi, Mikael Simons and Peter J. Cullen).
LCB team included senior researcher Lorena Simón-Gracia, Ph.D. student Allan Tobi, and Prof. Tambet Teesalu.
Neuropilin-1 is a host factor for SARS-CoV-2 infection“ (DOI: 10.1126/science.abd3072)
Neuropilin-1 facilitates SARS-CoV-2 cell entry and infectivity“ (DOI: 10.1126/science.abd2985)
Collaborative study shows that reprogramming tumor macrophages increases the efficacy of chemotherapy in breast cancer mice
Macrophages are immune system cells with a highly plastic phenotype. In established tumors, macrophages are skewed towards a pro-tumoral, anti-inflammatory, phenotype (M2 tumor associated macrophages, or “M2-TAMs”) by tumor-secreted cytokines and hypoxia.
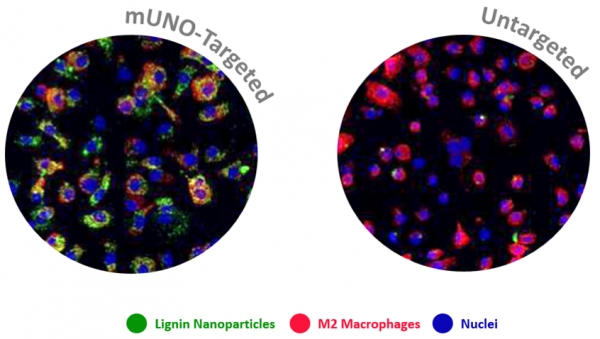
In this collaboration with the Helder Santos Lab (University of Helsinki, Finland), Figueiredo et al. showed that resiquimod was able to enhance chemotherapy in triple negative breast cancer only when it was targeted to M2-TAMs using the peptide "mUNO" identified in our lab. Resiquimod encapsulated in mUNO-coated lignin nanoparticles slowed down the tumor growth and significantly transformed the immune landscape in the tumor: decreased M2-TAM, increased CD8 T-cells, M1-TAM, and IFN-γ; these effects did not occur with free resiquimod or untargeted resiquimod-loaded nanoparticles
Anni Lepland, Pablo Scodeller and Tambet Teesalu from our lab participated in this collaboration, titled: "Peptide-guided resiquimod-loaded lignin nanoparticles convert tumor-associated macrophages from M2 to M1 phenotype for enhanced chemotherapy" https://doi.org/10.1016/j.actbio.2020.09.038
Special issue of Pharmaceutics: “Precision Delivery of Drugs and Imaging Agents with Peptides”
Prof. T. Teesalu and Dr. K. Kurrikoff (Institute of Technology, University of Tartu) act as guest editors of the special issue of Pharmaceutics (IF: 4.4) on peptide-based precision targeting. We invite experts around the world to submit their original research reports and reviews on preclinical and clinical applications of peptide-based targeting for delivery of molecular and nanoscale diagnostic and therapeutic payloads in the context of cancer, cardiovascular and pulmonary diseases, and regenerative medicine.
More information and instructions for submission: https://www.mdpi.com/journal/pharmaceutics/special_issues/precision_delivery
Submission deadline: January 31st 2021.
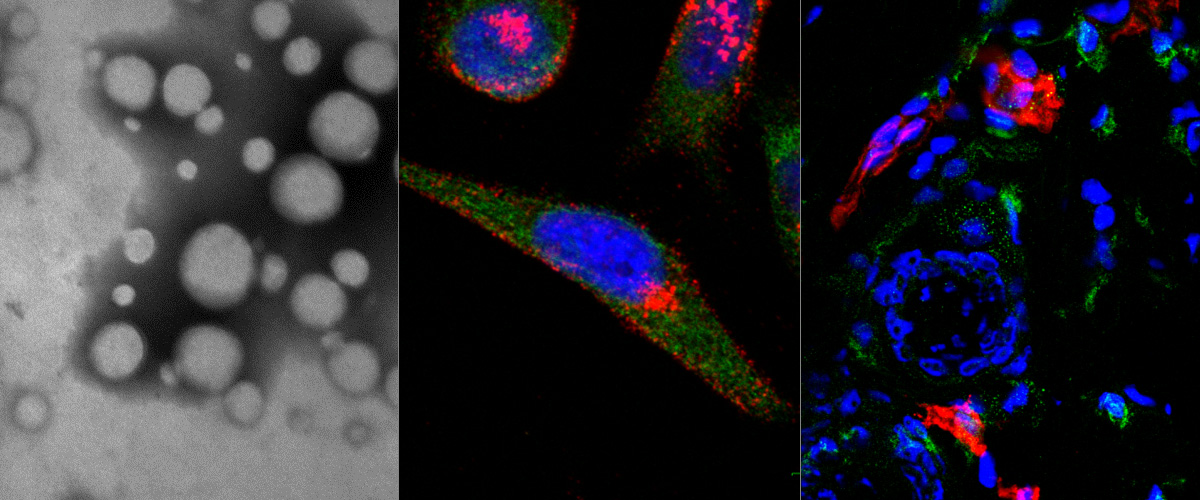
Silver Nanocarriers Targeted with a CendR Peptide Potentiate the Cytotoxic Activity of an Anticancer Drug
We report application of affinity-targeted silver nanoparticles (AgNPs) for selective delivery and potentiation of the activity of cytotoxic compound, monomethyl auristatin E (MMAE), in malignant cells. AgNPs were loaded with MMAE via a lysosomal protease cathepsin B sensitive linker and functionalized with a prototypic CendR peptide (RPARPAR) that targets neuropilin-1 (NRP-1) to give the AgNPs a dual tumor specificity as both cathepsin B and NRP-1 are overexpressed in many types of solid tumors. Cellular imaging, flow cytometry, viability assays and high performance liquid chromatography-mass spectrometry (HPLC-MS) analysis showed that the RPARPAR-MMAE-AgNPs are internalized and induce apoptotic cell death in NRP-1-positive PPC-1 prostate cancer cells while sparing NRP-1-negative M21 melanoma cells. The anticancer activity of precision target therapeutic AgNPs will be next studied in series of in vivo models, to determine the effect of nanoformulation and affinity targeting on the therapeutic index of MMAE.
This study, published in Advanced Therapeutics, is a sequel to a series of our studies on precision AgNPs. In addition to the contribution of the members of the Cancer Biology team ( PhD cand. Allan Tobi, Dr. Anne-Mari A. Willmore, PhD cand. Valeria Sidorenko and Prof. Tambet Teesalu) the study included contributions of our collaborators in the US (Prof. Erkki Ruoslahti and Ass. Prof. Kazuki N. Sugahara), Canada (Dr. Gary B Braun), and University of Tartu (Prof. Ursel Soomets and Dr. Kalle Kilk).
Journal Title - Advanced Therapeutics Article Title - Silver Nanocarriers Targeted with a CendR Peptide Potentiate the Cytotoxic Activity of an Anticancer Drug Manuscript ID - adtp.202000097 https://onlinelibrary.wiley.com/doi/10.1002/adtp.202000097
Uptake of nanoparticles functionalized with prototypic C-end Rule peptide RPARPAR-OH in cultured PPC-1 prostate carcinoma cells (movie: Tambet Teesalu)
Collaborative studies link CendR pathway to SARS Coronavirus-2 infection and spreading.
Our discovery of the CendR tissue transport pathway in 2009 raised the fascinating question of the physiological function of this pathway. It has become increasingly clear that the CendR pathway has been hijacked by viruses and microbial toxins for cell entry and tissue spreading. Cleavage of a viral surface proteins and protoxins by host proteases (most commonly furins and related enzymes) at sites that create an active CendR motif is a recurrent theme seen in many pathogens. Known examples include the Human T-lymphotropic virus-2,Crimean–Congo hemorrhagic fever virus, tick-born encephalitis virus, and Ebola viruses, as well as anthrax toxin.
In two collaborative studies with laboratories in Finland, Germany and UK; we showed that SARS-CoV-2 Spike protein S1 directly binds to the b1 domain of Neuropilin receptors on the cell surface. These studies also demonstrated that preventing this binding interaction resulted in decreased cell infection. This interaction suggests that neuropilins are important factors that modulate the infectivity of the SARS-CoV-2 viral entry in cells and target for therapeutic approaches to reduce SARS-CoV-2 viral infection.
These findings, published on the preprint server bioRxiv, are undergoing peer review. (http://doi.org/dx5d; 2020) (http://doi.org/dx5c; 2020).
Collaborative reports with Prof. Santos and Prof. Salonen in Finland and Prof. Mougdil in the USA.
Collaborative studies in interdisciplinary fields, such as nanomedicine, are critical for developing synergies and robust translationally relevant platforms.
Recently, our team participated in highly rewarding and productive collaborative research efforts with the research groups of Prof. Hélder Santos (on myocardial infarction targeting), Prof. Jarno Salonen (on development of novel hierarchically structured silicon nanoparticles) and Prof. Kamal Mougdil (on development of novel homing peptide that targets demyelinating neuroinflammatory lesions in brain). In these interdisciplinary studies, our researchers contributed their expertise in development of advanced peptidic targeting ligands and nanocarriers. We look forward for future successful joint research endeavors!
- Torrieri G, Fontana F, Figueiredo P, Liu Z, Ferreira MPA, Talman V, Martins JP, Fusciello M, Moslova K, Teesalu T, Cerullo V, Hirvonen J, Ruskoaho H, Balasubramanian V, Santos HA. Dual-peptide functionalized acetalated dextran-based nanoparticles for sequential targeting of macrophages during myocardial infarction. Nanoscale. 2020 Jan 28;12(4):2350-2358. https://pubmed.ncbi.nlm.nih.gov/31930241
- Mäkilä E, Anton Willmore AM, Yu H, Irri M, Aindow M, Teesalu T, Canham LT, Kolasinski KW, Salonen J. Hierarchical Nanostructuring of Porous Silicon with Electrochemical and Regenerative Electroless Etching. ACS Nano. 2019 Nov 26;13(11):13056-13064. https://pubmed.ncbi.nlm.nih.gov/31670505
- Acharya B, Meka RR, Venkatesha SH, Lees JR, Teesalu T, Moudgil KD. A novel CNS-homing peptide for targeting neuroinflammatory lesions in experimental autoimmune encephalomyelitis. Mol Cell Probes. 2020 Jun;51:101530. https://pubmed.ncbi.nlm.nih.gov/32035108
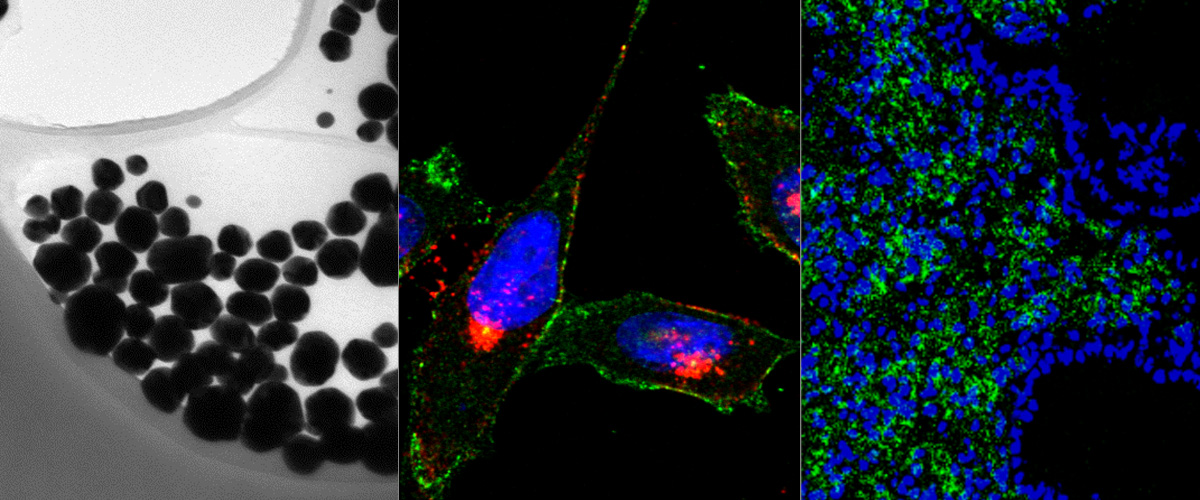
CendR peptide for Tenascin-C C domain isoform targeting.
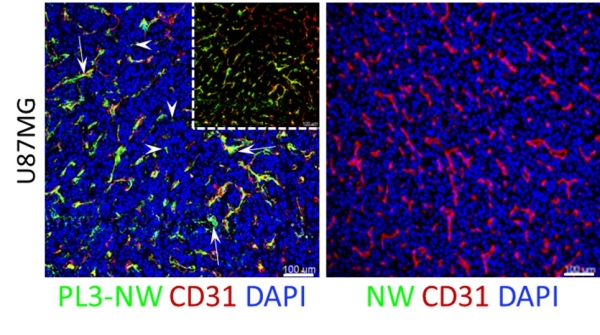
We describe in Scientific Reports a new homing peptide that interacts with the C-isoform of Tenascin-C (TNC-C) upregulated in malignant tissues and is capable of interacting via its C-end Rule (CendR) motif with cell-and tissue penetration receptor neuropilin-1 (NRP-1). We found that coating nanoparticles with PL3 peptide increased their tropism towards glioblastoma (GBM) and prostate carcinoma xenograft lesions in nude mice. Furthermore, treatment of glioma-bearing mice with proapoptotic PL3-guided NWs improved the survival of the mice, whereas treatment with untargeted particles had no effect. Importantly, PL3-coated nanoparticles accumulated in TNC-C and NRP-1-positive areas in clinical tumor samples, suggesting a translational relevance.
This work was performed in collaboration with the Department of Neurosurgery of Tartu University Clinics and Prof. Rolf Bjerkvig at the University of Bergen.
Lingasamy P, Tobi A, Kurm K, Kopanchuk S, Sudakov A, Salumäe M, Rätsep T,Asser T, Bjerkvig R, Teesalu T. Tumor-penetrating peptide for systemic targeting of Tenascin-C. Sci Rep. 2020 Apr 2;10(1):5809. DOI: 10.1038/s41598-020-62760-y
https://pubmed.ncbi.nlm.nih.gov/32242067
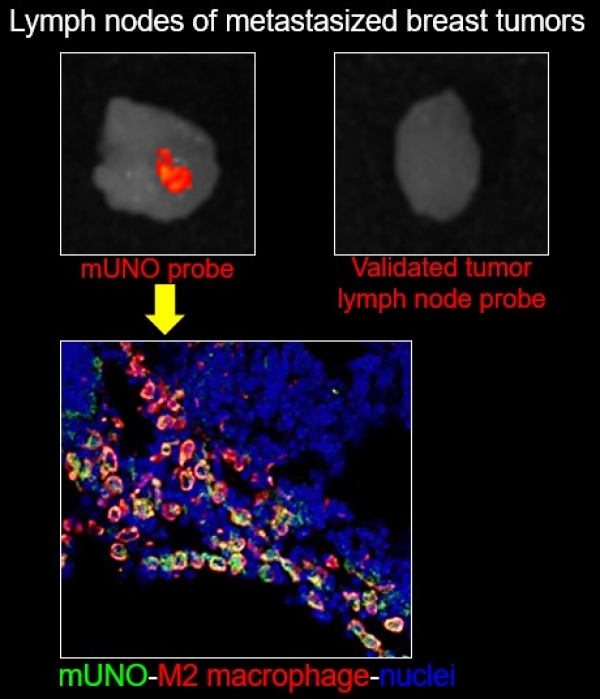
mUNO peptide targets M2-TAMs in primary and metastatic breast tumors.
Our collaborative study, published in Molecular Pharmaceutics describes the application of a short linear peptide (CSPGAK, "m(ini)UNO") for the delivery of molecular and nanoscale cargoes in M2-like tumor associated macrophages in vitro and the relevance of the peptide for in vivo targeting of early-stage primary breast tumors and metastatic lung foci.
This important study provides additional mechanistic understanding of mUNO binding and internalization pathway, addresses the effect of route of administration on the peptide bioavailability, and shows the robust accumulation of mUNO in M2-skewed macrophages in mouse models of early primary breast tumor and lung metastasis. This work also illustrates that mUNO can serve as a diagnostic probe for metastatic dissemination, by guiding an imaging agent to M2 macrophages in tumor and lymph node, more sensitively than an established tumor lymph node targeting agent.These studies support application of mUNO peptide for delivery of molecular and nanoscale cargoes to M2 macrophages in primary and metastatic breast tumors.
Lepland A, Asciutto EK, Malfanti A, Simón-Gracia L, Sidorenko V, Vicent MJ, Teesalu T, Scodeller P. Targeting Pro-Tumoral Macrophages in Early Primary and Metastatic Breast Tumors with the CD206-Binding mUNO Peptide. Mol Pharm. 2020 Jun 1. doi: 10.1021/acs.molpharmaceut.0c00226
https://pubmed.ncbi.nlm.nih.gov/32421341