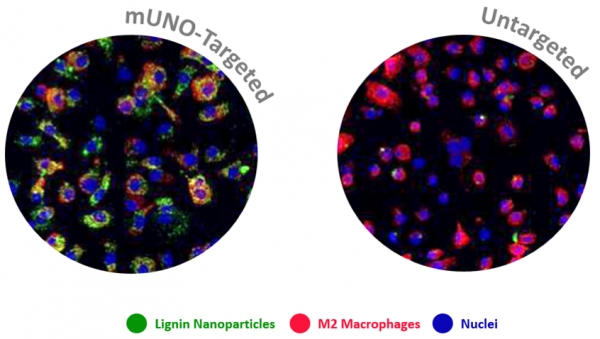
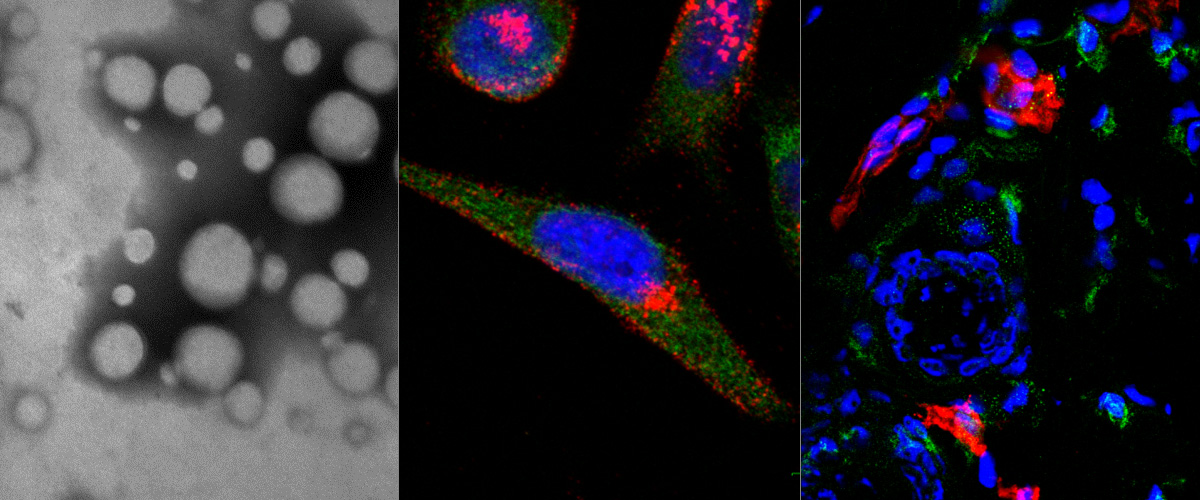
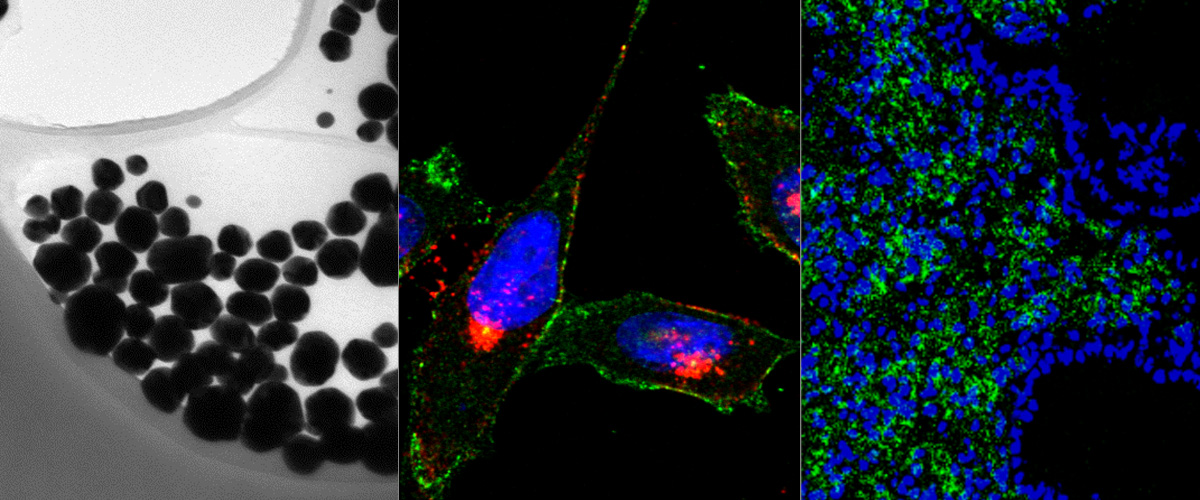
Macrophages are immune system cells with a highly plastic phenotype. In established tumors, macrophages are skewed towards a pro-tumoral, anti-inflammatory, phenotype (M2 tumor associated macrophages, or “M2-TAMs”) by tumor-secreted cytokines and hypoxia.
In this collaboration with the Helder Santos Lab (University of Helsinki, Finland), Figueiredo et al. showed that resiquimod was able to enhance chemotherapy in triple negative breast cancer only when it was targeted to M2-TAMs using the peptide "mUNO" identified in our lab. Resiquimod encapsulated in mUNO-coated lignin nanoparticles slowed down the tumor growth and significantly transformed the immune landscape in the tumor: decreased M2-TAM, increased CD8 T-cells, M1-TAM, and IFN-γ; these effects did not occur with free resiquimod or untargeted resiquimod-loaded nanoparticles
Anni Lepland, Pablo Scodeller and Tambet Teesalu from our lab participated in this collaboration, titled: "Peptide-guided resiquimod-loaded lignin nanoparticles convert tumor-associated macrophages from M2 to M1 phenotype for enhanced chemotherapy" https://doi.org/10.1016/j.actbio.2020.09.038