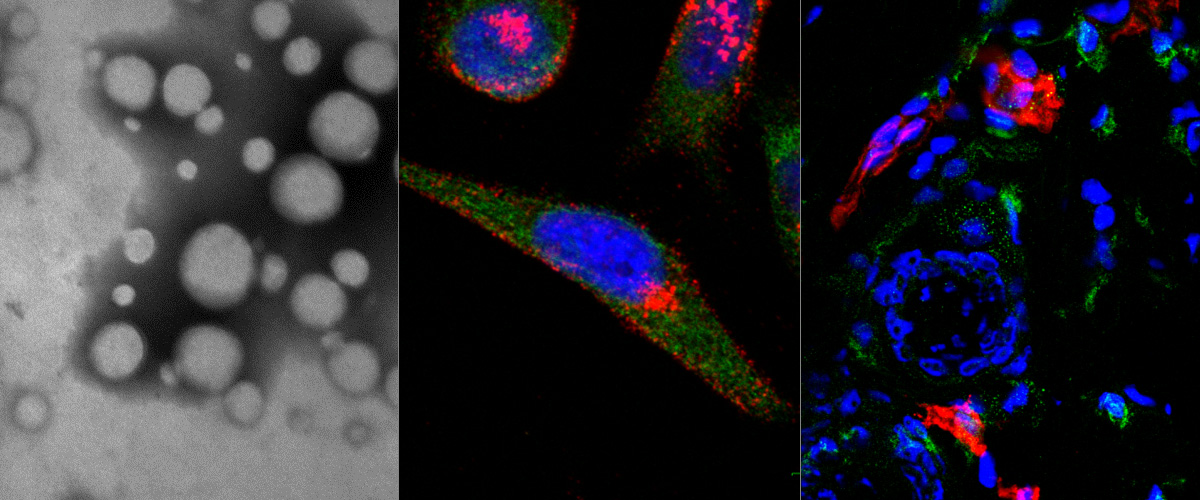
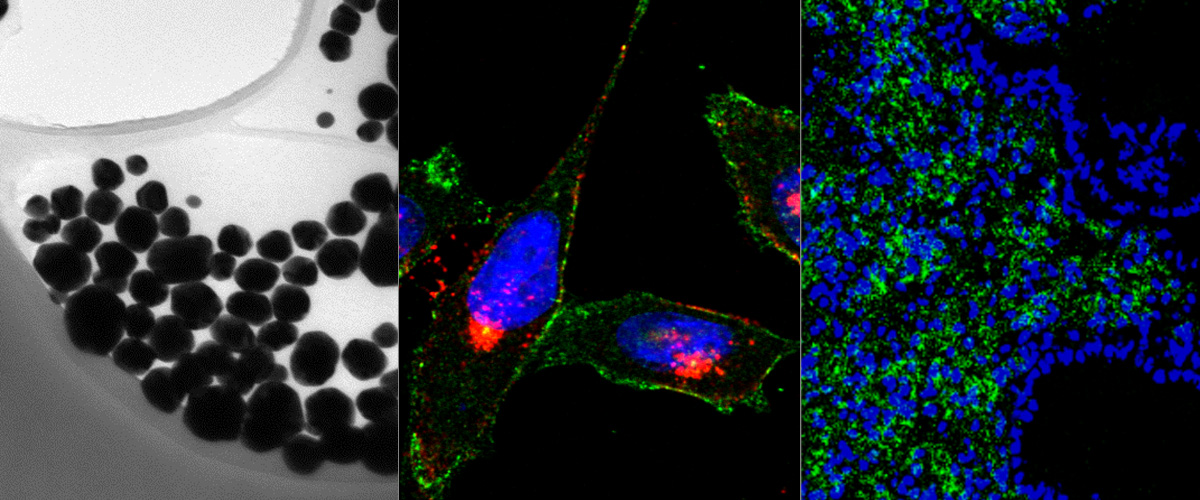
Our discovery of the CendR tissue transport pathway in 2009 raised the fascinating question of the physiological function of this pathway. It has become increasingly clear that the CendR pathway has been hijacked by viruses and microbial toxins for cell entry and tissue spreading. Cleavage of a viral surface proteins and protoxins by host proteases (most commonly furins and related enzymes) at sites that create an active CendR motif is a recurrent theme seen in many pathogens. Known examples include the Human T-lymphotropic virus-2,Crimean–Congo hemorrhagic fever virus, tick-born encephalitis virus, and Ebola viruses, as well as anthrax toxin.
In two collaborative studies with laboratories in Finland, Germany and UK; we showed that SARS-CoV-2 Spike protein S1 directly binds to the b1 domain of Neuropilin receptors on the cell surface. These studies also demonstrated that preventing this binding interaction resulted in decreased cell infection. This interaction suggests that neuropilins are important factors that modulate the infectivity of the SARS-CoV-2 viral entry in cells and target for therapeutic approaches to reduce SARS-CoV-2 viral infection.
These findings, published on the preprint server bioRxiv, are undergoing peer review. (http://doi.org/dx5d; 2020) (http://doi.org/dx5c; 2020).