News
Review on affinity targeting of peritoneal carcinomatosis
Lorena, Hedi and Tambet from LCB published a review entitled „Peritoneal Carcinomatosis Targeting with Tumor Homing Peptides“ in Molecules (2018, 23(5), 1190; https://doi.org/10.3390/molecules23051190). The review summarizes progress from our and other research labs on affinity targeting of intraperitoneal anticancer compounds, imaging agents and nanoparticles with tumor-homing peptides. We review classes of tumor-homing peptides relevant for PC targeting, payloads for peptide-guided precision delivery, applications for targeted compounds, and the effects of nanoformulation of drugs and imaging agents on affinity-based tumor delivery.
Research Day of the Department of Biomedicine (March 19, 2018)
Lab of cancer biology and other groups of the Deparment of Biomedicine (groups of Molecular Pathology, RNA Biology, and Human Genetics) had a day full of stimulating presentations and academic exchange at the beautiful Võrtsjärve Research Station/Lake Museum. Presenters from our laboratory were Anett Laarmann (Development of tumor penetrating antibodies), Anni Lepland (Therapeutic precision targeting of tumor-associated macrophages), Prakash Lingasamy (Dual-specific tumor targeting novel peptide for drug delivery), and Valeria Sidorenko (Smart nanoparticles for breast cancer detection and therapy).

Successful grant applications
Successful grant applications
Dr. Teesalu was awarded two prestigious grants:
(1) ~1M EUR Estonian Research Council grant to peptide-based develop transport systems to cross blood brain barrier

(2) 150.000 EUR European Research Council proof of concept grant to develop precision glioblastoma nanotherapies (the first ever ERC proof of concept grant to be awarded to an Estonian researcher).

In addition, Dr. Teesalu established a partnership in EUR 700.000 industrial collaboration project to develop affinity ligand-drug conjugates for precision cancer therapy with Toxinvent LLC (Tartu, Estonia).

Congratulations to Anne-Mari for successfully defending her PhD thesis!
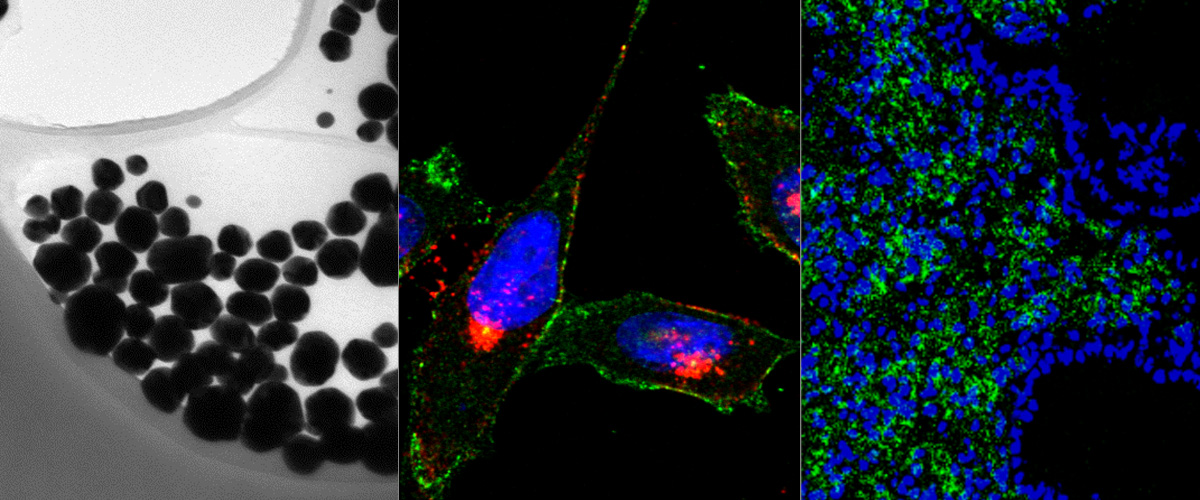
Anne-Mari Anton Willmore successfully defended her Ph.D. thesis „Silver nanoparticles for cancer research“ on November 17th, 2917. Her thesis dealt with the development of silver nanoparticles as a cancer research tool and potential nanocarrier for targeted tumor delivery.
https://www.ut.ee/en/events/anne-mari-anton-willmore-silver-nanoparticles-cancer-research
Image: Anne Mari Anton Willmore and her opponent Professor Hélder A. Santos (D.Sc. Tech.) of the University of Helsinki (Finland) during the thesis discussion.
A peptide homing to early lesions of Alzheimer's disease reported in Nature Communications
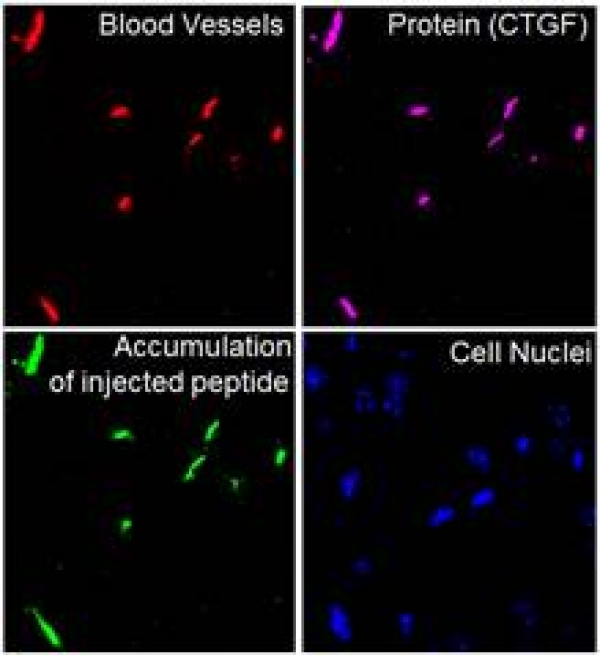
The Ruoslahti Lab in La Jolla, in collaboration with our lab, has found a biomarker of early Alzheimer's disease (AD). This biomarker, identified as the protein Connective tissue growth factor (CTGF) is deposited on the brain blood vessels of early AD mice. We have also identified a peptide (named DAG) that recognizes CTGF and homes to early AD mice, well before the appearance of amyloid beta plaques. This peptide can take with it iron oxide nanoparticles that can serve as contrast in Magnetic Resonance Imaging of AD lesions.
Link to the paper: https://www.nature.com/articles/s41467-017-01096-0
Link to the news coverage: Cancer biologists from the University of Tartu help make an important discovery on Alzheimer’s disease
Image: Brain of a mouse with early Alzheimer's disease injected with green fluorescent DAG peptide.. The target protein, CTGF (magenta), appears on the blood vessels (red) of AD brains much before the appearance of amyloid beta plaques. The DAG peptide (green) specifically recognizes CTGF on these blood vessels upon intravenous administration. Credit: Pablo Scodeller and Aman Mann.
Our M2 Tumor macrophage targeting peptide published in Scientific reports!
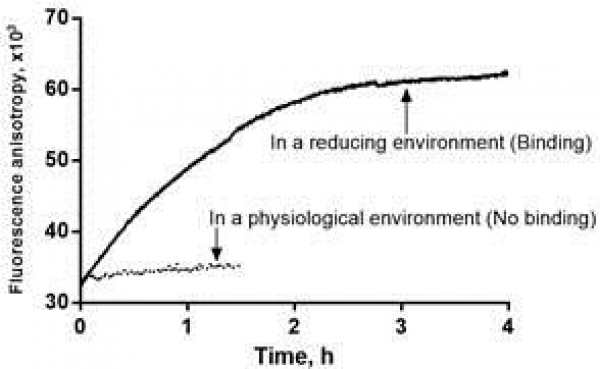
We have identified a peptide (nicknamed "UNO") that internalizes in M2 tumor associated macrophages (TAMs) by binding to CD206. Systemically administered UNO homed to M2 TAMs in 5 differerent solid tumor models and was able to carry with it a fluorescent payload and nanoscale polymeric vesicles, polymersomes. Importantly, the cyclic UNO peptide preferentially recognizes CD206 in the tumor and not in the healthy tissues, as it is activated by the reducing environment found in the tumor microenvironment.
Link to the paper: https://www.nature.com/articles/s41598-017-14709-x
Image: Cell-free interation of UNO with recombinant CD206. Increase of anisotropy indicates binding of the peptide to CD206. Credit: Pablo Scodeller and Sergei Kopanchuk.
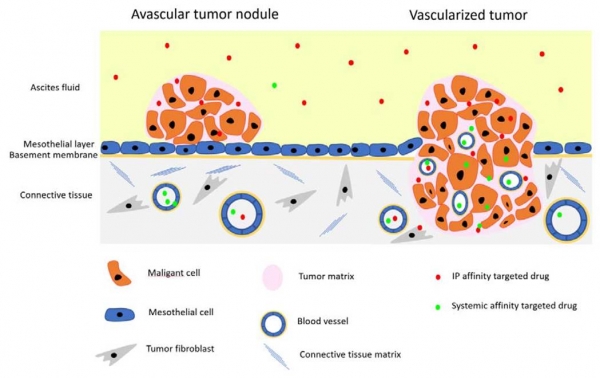
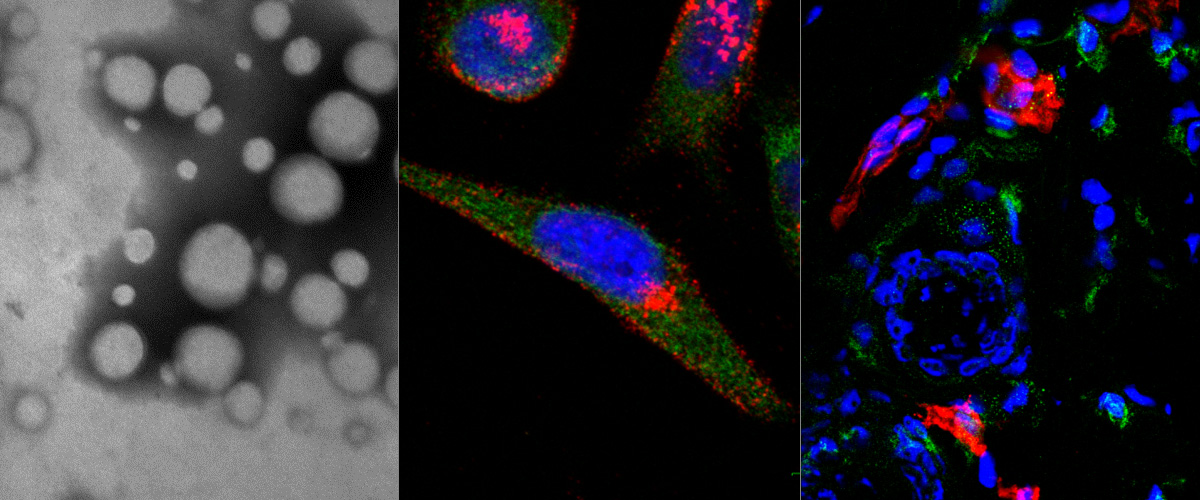
Targeting of p32 in peritoneal carcinomatosis with intraperitoneal linTT1 peptide-guided pro-apoptotic nanoparticles
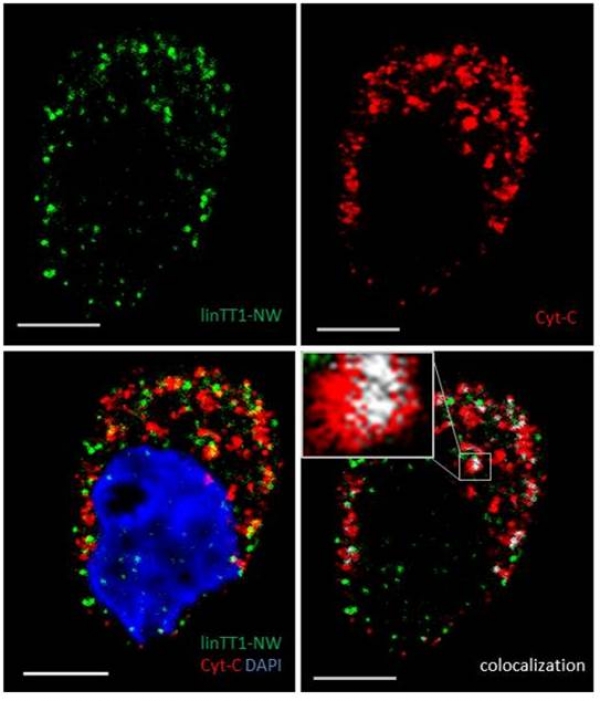
We report in the Journal of Controlled Release application of a novel tumor penetrating peptide, linTT1 (AKRGARSTA) for targeting nanoparticles for the detection and treatment of peritoneal carcinomatosis. Iron oxide nanoworms (NWs) functionalized with the linTT1 peptide were taken up and routed to mitochondria in cultured peritoneal carcinomatosis cells. NWs functionalized with linTT1 peptide in tandem with a pro-apoptotic [D(KLAKLAK)2] peptide showed p32-dependent cytotoxicity in MKN-45P, SKOV-3, and CT-26 cells. Upon IP administration in mice bearing MKN-45P, SKOV-3, and CT-26 tumors, linTT1-functionalized NWs showed robust homing and penetration into malignant lesions, whereas only a background accumulation was seen in control tissues. Finally, experimental therapy of mice bearing peritoneal MKN-45P xenografts and CT-26 syngeneic tumors with IP linTT1-D(KLAKLAK)2-NWs resulted in significant reduction of weight of peritoneal tumors and significant decrease in the number of metastatic tumor nodules, whereas treatment with untargeted D(KLAKLAK)2-NWs had no effect. Our findings suggest that linTT1-targeted nanoparticles may potentially be translated to therapeutic interventions against peritoneal carcinomatosis.
Targeting of p32 in peritoneal carcinomatosis with intraperitoneal linTT1 peptide-guided pro-apoptotic nanoparticles. Hunt H, Simón-Gracia L, Tobi A, Kotamraju VR, Sharma S, Nigul M, Sugahara KN, Ruoslahti E, Teesalu T.J Control Release. 2017 Aug 28;260:142-153. doi: 10.1016/j.jconrel.2017.06.005. Epub 2017 Jun 8. PMID:28603028
Image (Hedi Hunt M.Sc.): Internalized linTT1-NWs colocalize with a mitochondrial marker, cytochrome C in cultured MKN-45P cells. linTT1-NW: green; cytochrome C (Cyt-C): red; DAPI: blue; colocalization of FAM and cytochrome C signal: white. Scale bar: 5 mm.
Ratiometric in vivo auditioning of targeted silver nanoparticles
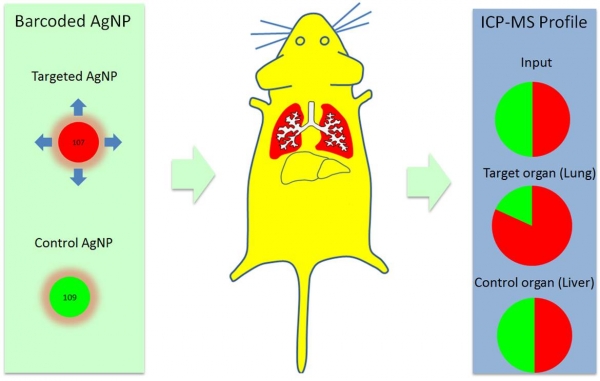
We report in the Nanoscale the in vivo application of the technology of isotopically-barcoded ratiometric silver nanoparticles for quantitative biodistribution studies. In a proof of concept study we used peptides with previously described tissue tropism; one peptide that favors vascular beds of the normal lungs (RPARPAR; receptor neuropilin-1, or NRP-1) and another that is selective for central nervous system vessels (CAGALCY). Equimolar mixtures of the peptide-targeted Ag107-NPs and Ag109 control particles were mixed and injected intravenously. Distribution profiles of Ag107 and Ag109 in tissue extracts were determined simultaneously through inductively coupled plasma mass spectrometry (ICP-MS, both on tissue extracts and on cryosections to obtain spatial information). Internally controlled ratiometric AgNP system appears to be suitable for quantitative studies of the effect of targeting ligands on NP biodistribution, at average tissue concentration and distribution at the microscopic level. The platform might be particularly relevant for target sites with high local variability in uptake, such as tumors.
Schematic representation of the concept of isotopically-barcoded silver nanoparticles for in vivo biodistribution studies (Tambet Teesalu).
Ratiometric in vivo auditioning of targeted silver nanoparticles.Toome K, Willmore AA, Paiste P, Tobi A, Sugahara KN, Kirsimäe K, Ruoslahti E, Braun GB, Teesalu T.Nanoscale. 2017 Jul 20;9(28):10094-10100. doi: 10.1039/c7nr04056c. PMID: 28695222
Targeted nanosystems for imaging and therapy Spring workshop, Tartu, Estonia, May 10-12, 2017
This workshop provides an opportunity to get an overview of translational nanobiomedicine research from the leading European experts. The overarching themes are interactions of nanoparticles with biological systems, applications of precision-guided nanosystems for imaging and more efficient therapies, and the relationship of nanotechnology with personalized medicine.
Targeted nanosystems for imaging and therapy
Spring workshop, Tartu (Estonia)
May 10-12, 2017
Venue: V-Spa Hotel and Conference Center, Riia 2, Tartu
Target audience : Graduate students , research staff
Organizers: Lorena Simón Gracia This email address is being protected from spambots. You need JavaScript enabled to view it.
Tambet Teesalu This email address is being protected from spambots. You need JavaScript enabled to view it.
Free Registration at: http://registration.amarela.ee/spring-workshop/
Program and more information : Click Here
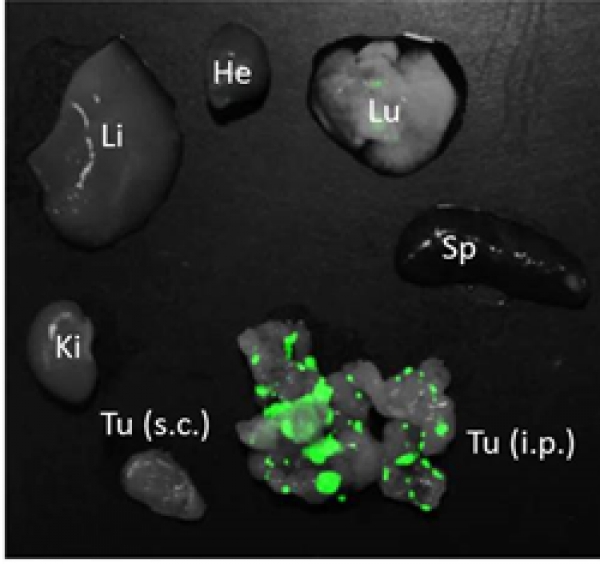
iRGD-targeted polymersomes for enhanced intraperitoneal chemotherapy
We report in Biomaterials development of a tumor-specific delivery system for the treatment of peritoneal carcinomatosis. We demonstrate that after intraperitoneal administration, pH-sensitive polymeric vesicles loaded with an anticancer drug paclitaxel and functionalized with the tumor penetrating peptide iRGD specifically accumulate in peritoneal tumors in mice and have higher antitumor activity than free paclitaxel or Abraxane (a nano-formulation currently used in the therapy of several types of carcinoma). Our findings suggest that iRGD polymersomes may potentially be translated to therapeutic interventions against peritoneal carcinomatosis.
This collaborative study was driven and coordinated by our senior researcher Lorena Simón-Gracia and carried out together with Prof. Giuseppe Battaglia’s lab in UC London (UK) and with Drs. Kazuki N Sugahara and Ramana Kotamraju and Prof. Erkki Ruoslahti at Sanford Burnham Prebys Medical Discovery Institute in La Jolla, Calif. (USA).
iRGD peptide conjugation potentiates intraperitoneal tumor delivery of paclitaxel with polymersomes Biomaterials. 2016 Jul 20;104:247-257.
Image: Homing of green fluorescent iRGD polymersomes in CT26 peritoneal tumor (Tu). Note that control organs (liver, Li; lung, Lu; spleen, Sp; kidney, Ki) and subcutaneous tumors (Tu s.c.) show minimal labeling.