News
Continued success in Euronanomed grant applications: ECM-CART project funded.
We are pleased to announce that our success in applying for Euronanomed grants continues. In November of 2021 it was announced that application “LDL-like nanoparticles for CAR-T-based glioblastoma immunotherapy” by our consortium led by Dr. Pablo Hervella (Instituto de Investigación Sanitaria de Santiago de Compostela, Spain) was successful. This project will seek to demonstrate that the targeted delivery of exogenous antigens to the extracellular matrix (ECM) of brain tumors provides a safe and specific target for chimeric antigen receptor T- cell (CART) for glioblastoma (GBM) treatment.
PNAS perspective: a widespread relevance of C-end Rule peptides to infection by different viruses.
In November of 2020 our team participated in two high-profile studies in Science that demonstrated that as SARS-CoV2 coronavirus matures, an enzymatic event causes the virus to present a specific peptide motif (C-end Rule or CendR peptide), and makes it accessible to bind with a widely-expressed class of mammalian NRP receptors. These reports focused on experiments to rigorously demonstrate the link between SARS-CoV2 and NRP-1, and we did not have sufficient space to discuss what we see as a fascinating and impactful emerging theme, namely that the NRP-1 pathway appears to influence viral infections in a generalized and widely-applicable manner.
In the current PNAS Perspective entitled “A widespread viral entry mechanism: The C-end Rule motif-neuropilin receptor interaction”, together with Drs Giuseppe Balistreri and Yohei Yamauchi (virologists at University of Helsinki and Bristol, respectively) we outline data-driven hypothesis and “connect the dots” to suggest that it is possible (and, indeed, likely) that NRP plays a critical role in infectivity and spread of multiple human pathogenic viruses. This model is seemingly relevant for any virus whose life cycle includes processing by the furin family of proteases, and that list includes a wide range of viruses that pose significant global health concerns, such as Influenza H5N1, Ebola, Dengue, Zika, West Nile, Yellow fever virus, Human Immunodeficiency virus, Hepatitis B virus, and Human Papilloma Virus.
An essential therapeutic implication of this concept is that a generalized approach to elicit pharmacological interference with the CendR/NRP-1 axis may offer an opportunity to attenuate the infectivity of all of these viruses!
We continue working on development of the further insight into the CendR/virus axis.
Reference: Proc Natl Acad Sci U S A, . 2021 Nov 16;118(49):e2112457118. A widespread viral entry mechanism: The C-end Rule motif-neuropilin receptor interaction. Giuseppe Balistreri, Yohei Yamauchi, Tambet Teesalu
Congratulations to our PhD students for prestigious prizes!
The fall of 2021 has been very successful for our PhD students - Valeria Sidorenko, Allan Tobi and Kristina Põšnograjeva received prestigious grants from the Foundation of University of Tartu.
Valeria received a memorial scholarship from Valda and Bernard Õun Memorial Foundation in the amount of 5000 euros for attending an international conference.
Allan received the Hilda and Harry Mägi stipend for the amount of 2000€ to support further research and studies.
Kristina received a stipend of 2500 euros from the memorial fund of Liisa Kolumbus that she will use to attend an international conference.
Congratulations to our talented PhD students!
Science day of Department of Biomedicine in Sangaste castle (June 17, 2021)
The research labs of the Department of Biomedicine (Molecular Pathology, Molecular Genetics, RNA Biology, and Precision and Nanomedicine) had a traditional joint Science Day on which graduate students reported on the progress of their projects. The event was held in beautiful Sangaste castle and was full of stimulating talks and Q&A sessions. Presentation by our PhD student Kristina Põšnograjeva “Searching for blood brain barrier penetrating peptides” was voted the winner of the best presentation prize for the best presentation. Congratulations, Kristina!!!
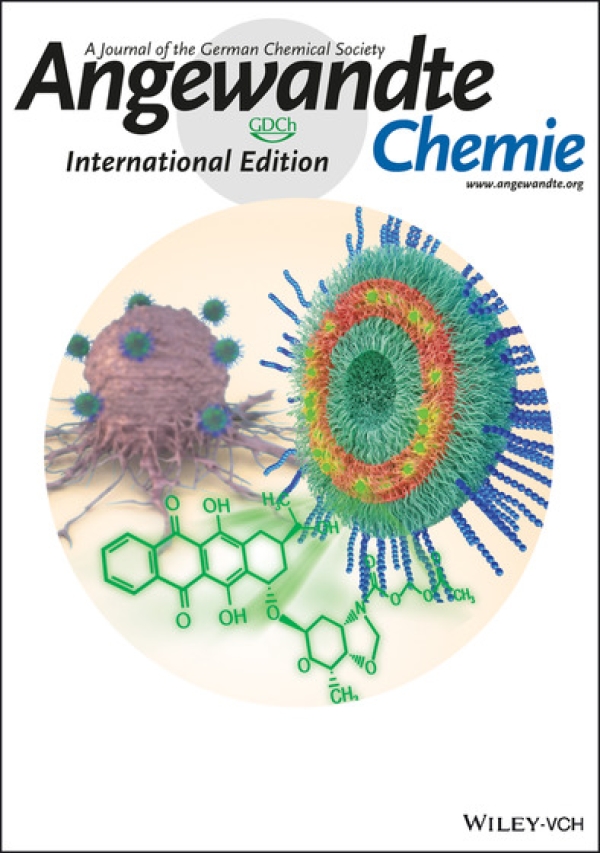
Development of novel anthracycline utorubicin for cancer therapy reported in Angewandte Chemie.
In a study reported in Angewandte Chemie we report the development of Utorubicin (UTO) as a potent anticancer payload of polymeric nanoparticles. Nanoencapsulation of UTO in polymeric vesicles functionalized with tumor-penetrating peptides increases the cytotoxicity of the drug in receptor-positive cells in vitro and, after systemic administration into mice bearing triple-negative tumors, potentiates UTO accumulation in malignant tissue.
This collaborative study was led by team of Precision and nanomedicine (Lorena Simón-Gracia, Valeria Sidorenko, Tambet Teesalu) and involved Estonian drug development company Toxinvent Inc.
Links:
- Novel Anthracycline Utorubicin for Cancer Therapy, Angew Chem, 2021 Jul 26;60(31):17018-17027. https://onlinelibrary.wiley.com/doi/10.1002/anie.202016421
- Nanoparticles Could Help to Deliver Cancer-Drug to the Right Target, https://researchinestonia.eu/2018/12/20/nanoparticles-could-help-to-deliver-cancer-drug-to-the-right-target/
- Smart specialisation project “Innovative anticancer drug candidates based on a novel cytotoxic compound, Utorubicin“
Internationally recognized nanomedicine expert Prof. Hélder A. Santos becomes Visiting Professor at our laboratory
With the global benefits of the new science of nanomedicine growing each year, the University of Tartu is determined to stay at the forefront of the international collaborative research in this area.
We are pleased to announce that outstanding researcher, educator, and raising star in nanomedicine, Prof. Hélder A. Santos, joins the Laboratory of Precision and Nanomedicine of University of Tartu as a Visiting Professor (effective February 1st, appointment for 2 years).
Prof Santos is a physical chemist specialized in electrochemistry, biological mimetic models and soft interfaces. His current work bridges engineering, pharmaceutical and medical research. His research focus is in the use of biodegradable and biocompatible nanoporous silicon nanomaterials, polymers, the application of microfluidics technology for nanoparticle production for controlled drug delivery, diagnosis and treatment of cancer, diabetes, and cardiovascular diseases, and further clinical translation of these nanotechnologies. Currently, is a Full Professor in Pharmaceutical Nanotechnology, Head of the Nanomedicines and Biomedical Engineering Group, and Director of the Doctoral Program in Drug Research at the Faculty of Pharmacy, at the University of Helsinki. He is also a Fellow Member of Helsinki Institute of Life Science (HiLIFE), the Director of the FinPharmaNet (The Network of Drug Research Doctoral Programmes in Finland), Chair of Controlled Release Society Focus Group in Nanomedicine and Nanoscale Delivery, and Chairman and co-founder of Capsamedix Oy.
The involvement of Prof. Santos will further strengthen the collaborative ties between the research labs and institutions to further facilitate collaborative efforts in translational nanomedicine and training of next generation of nanomedicine experts.
https://scholar.google.com/citations?user=K3Pj_gwAAAAJ&hl=fi
Contact info: This email address is being protected from spambots. You need JavaScript enabled to view it.
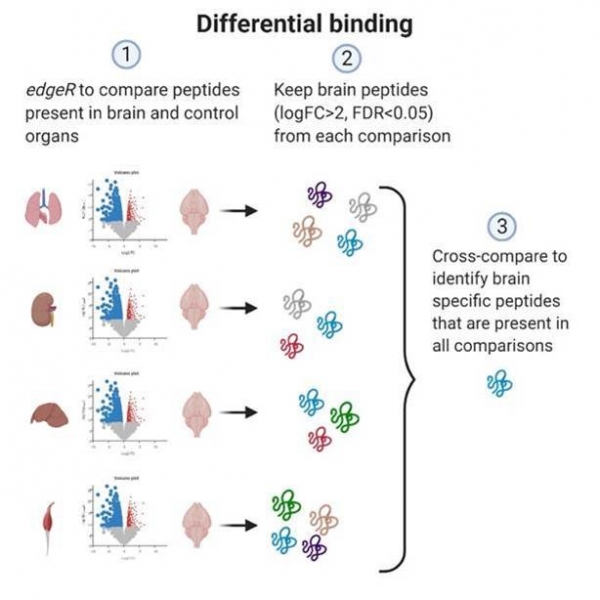
New tools to increase the reliability of in vivo phage biopanning reported in Nucleic Acids Research
In vivo phage display is widely used for the identification of organ- or disease-specific homing peptides. In our study (lead authors PhD students Karlis Pleiko and Kristina Põšnograjeva), we developed new tools to streamline in vivo biopanning screens. Our approach allows differential mapping of homing ability and organ-selectivity of peptide-displaying phages during in vivo selection based solely on the high throughput sequencing data. This study will accelerate mapping of vascular heterogeneity in vivo and, ultimately, catalyze progress toward development of affinity-guided smart drugs and contrast agents.
“In vivo phage display: identification of organ-specific peptides using deep sequencing and differential profiling across tissues”, Pleiko K, Põšnograjeva K, Haugas M, Paiste P, Tobi A, Kurm K, Riekstina U, Teesalu T; Nucleic Acids Research, (2021 Jan 14; https://doi.org/10.1093/nar/gkaa1279); “;
https://academic.oup.com/nar/advance-article/doi/10.1093/nar/gkaa1279/6097687
Congratulations to Prakash Lingasamy for successfully defending his PhD thesis!
Prakash Lingasamy successfully defended his Ph.D. thesis " Development of multitargeted tumor penetrating peptides" on January 29th, 2021. Opponent of the thesis was professor Angelo Corti from Università Vita-Salute San Raffaele (Milan, Italy). Compared to conventional monospecific tumor homing peptides, multispecific peptides identified in the thesis provide a better accumulation of therapeutic and diagnostic nanoparticles in solid tumors. These preclinical results warrant downstream clinical studies on development of peptide-guided drugs and imaging agents. We wish Prakash good luck in his future career!
https://dspace.ut.ee/handle/10062/70738
https://dspace.ut.ee/bitstream/handle/10062/70738/lingasamy_prakash.pdf
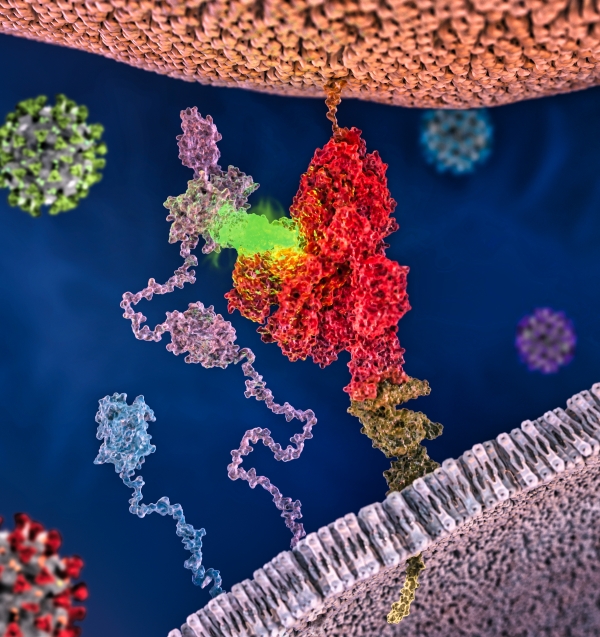
Two back-to-back collaborative studies published in Science on Neuropilin as a novel coronavirus receptor
Our lab was involved in two studies by international research teams that show that new coronavirus uses NRP-1 as a cellular internalization receptor.
The research stems from our earlier work on C-end Rule (CendR) peptides. CendR peptides act as position-dependent cellular switches: when present at C-terminus of any protein, they bind to Neuropilins to trigger cellular uptake and tissue penetration. The SARS-CoV-2 virus uses a protein called Spike to bind and enter host cells. New research shows that proteolytic processing of Spike protein by cellular enzyme furin results in activation of cryptic CendR peptide to trigger cellular uptake and tissue penetration of the CoV2 particles.
This discovery opens a new path for development of coronavirus drugs. Intriguingly, the CendR sequences are present on the surface of other pathogenic viruses, such as Ebola and HIV-1.
Our laboratory provided key reagents related to CendR pathway (antibodies, recombinant proteins, CendR nanoparticles) to the virology labs in Finland, UK, and Germany that coordinated the collaboration (PIs: Drs. Giuseppe Balistreri, Yohei Yamauchi, Mikael Simons and Peter J. Cullen).
LCB team included senior researcher Lorena Simón-Gracia, Ph.D. student Allan Tobi, and Prof. Tambet Teesalu.
Neuropilin-1 is a host factor for SARS-CoV-2 infection“ (DOI: 10.1126/science.abd3072)
Neuropilin-1 facilitates SARS-CoV-2 cell entry and infectivity“ (DOI: 10.1126/science.abd2985)
Collaborative study shows that reprogramming tumor macrophages increases the efficacy of chemotherapy in breast cancer mice
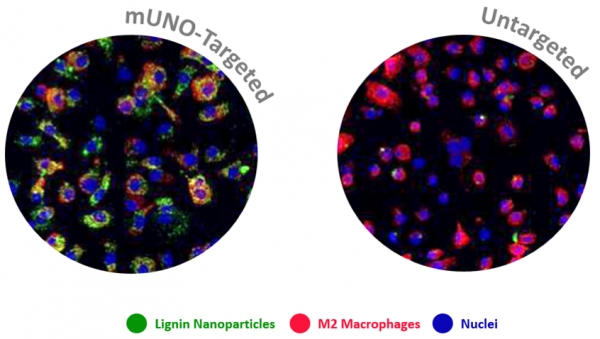
Macrophages are immune system cells with a highly plastic phenotype. In established tumors, macrophages are skewed towards a pro-tumoral, anti-inflammatory, phenotype (M2 tumor associated macrophages, or “M2-TAMs”) by tumor-secreted cytokines and hypoxia.
In this collaboration with the Helder Santos Lab (University of Helsinki, Finland), Figueiredo et al. showed that resiquimod was able to enhance chemotherapy in triple negative breast cancer only when it was targeted to M2-TAMs using the peptide "mUNO" identified in our lab. Resiquimod encapsulated in mUNO-coated lignin nanoparticles slowed down the tumor growth and significantly transformed the immune landscape in the tumor: decreased M2-TAM, increased CD8 T-cells, M1-TAM, and IFN-γ; these effects did not occur with free resiquimod or untargeted resiquimod-loaded nanoparticles
Anni Lepland, Pablo Scodeller and Tambet Teesalu from our lab participated in this collaboration, titled: "Peptide-guided resiquimod-loaded lignin nanoparticles convert tumor-associated macrophages from M2 to M1 phenotype for enhanced chemotherapy" https://doi.org/10.1016/j.actbio.2020.09.038