News
Looking forward to exciting 2024
Our lab is buzzing with activity, fueled by new grants and promising projects set to unfold in 2024. We eagerly await the publication of groundbreaking research and the filing of patent applications. The collaborative spirit within our team enhances creativity, fostering an environment where diverse talents converge to push the boundaries of scientific exploration. With an eye on both academic contributions and real-world applications, we're poised for a dynamic year of innovation and impactful discoveries.
Success: Transcan-3 Grant Awarded to "ReachGLIO" Project
We are thrilled to share the exciting news of our latest achievement in securing European funding. The consortium led by Prof. Pilar Sanchez Gomez from Unidad de Neurooncología, Instituto de Salud Carlos III, Majadahonda, Spain, has successfully obtained support for the project titled "Reaching the heterogeneous vascular landscape of glioblastoma with multifunctional nanomedicines." The Transcan-3 grant application was not only approved but also activated in late 2023.
The "ReachGLIO" project is poised to pave the way for a groundbreaking pre-clinical framework, essential for the development of a novel and effective therapy targeting high-grade gliomas. The approach involves the utilization of dual-targeted nanoparticles loaded with potent anti-cancer drugs. Furthermore, the project explores the potential synergy of this approach when combined with peptide-guided tumor necrosis factor.
This success marks a significant milestone in our pursuit of advancing cancer therapeutics. The funded project will contribute valuable insights and data, bringing us one step closer to a more effective treatment for glioblastomas. We express our gratitude to the Transcan-3 grant program for recognizing the potential impact of our research and supporting our efforts to combat high-grade gliomas.
Advancing Innovation: IP Protection Milestones in 2023
In 2023, our laboratory has been actively engaged in securing intellectual property (IP) for the innovative inventions developed within our research initiatives. We are pleased to announce significant progress in this endeavor, marked by the submission of two patent applications:
"BRAIN PENETRATING PEPTIDES AND METHODS OF USE THEREOF", Authors: Tambet Teesalu, Kristina Põšnograjeva, Maarja Haugas, Kadri Toome, patent application submitted in August 2023.
"PEPTIDES TARGETING INFECTED TISSUES AND METHODS OF USE THEREOF", US patent application submitted in May 2023.
These patent applications represent our commitment to translating cutting-edge research into tangible solutions. As a research and development institution, the University of Tartu continues to foster an environment that encourages innovation and the protection of intellectual property. Our ongoing commitment to protecting intellectual property ensures that the fruits of our labor have the potential to make a lasting impact on society.
Laboratory of Precision and Nanomedicine Spotlighted in Estonian Daily Eesti Päevaleht
Our Laboratory of Precision and Nanomedicine is proud to announce a recent feature article in the Estonian daily newspaper Eesti Päevaleht and news portal Delfi on October 11, 2023. The article delves into the impactful research conducted at the lab and explores the potential clinical implications of our work in the field of anticancer drug development.
In the feature, Professor Tambet Teesalu offers a comprehensive overview of the ongoing research initiatives within the laboratory. He sheds light on the innovative approaches being explored and discusses how these endeavors could lead to the development of more efficacious and better-tolerated anticancer drugs.
For a detailed insight into the interview and to explore the groundbreaking advancements in cancer treatment technology, you can read the full article here: Estonian Daily Eesti Päevaleht Feature.
We extend our gratitude to Eesti Päevaleht/Delfi for highlighting our research efforts, and we remain committed to pushing the boundaries of precision and nanomedicine for the advancement of cancer therapeutics. Stay tuned for more updates on our exciting journey.
New Faces and Exciting Projects: Welcoming Researchers to Our Team in 2023
In the dynamic landscape of 2023, our team has grown stronger with the addition of talented researchers, each bringing unique skills and perspectives to our collaborative efforts.
Antonella Rocchi MSc: Antonella joined us as a visiting PhD student from Prof. Christian Celia’s lab at the University of Chieti G. D’Annunzio / University of L’Aquila, Italy. Her focus lies in the development of peptide-guided nanoparticles for the treatment of the deadly brain tumor, glioblastoma. We are excited to benefit from her expertise and international collaboration.
Jorgen Holm MSc and Jhalak Sethi MSc: These two dynamic individuals have embarked on their PhD journeys with us in fall 2023. Their projects center around the development of homing peptides capable of targeting infiltrative glioma lesions in the brain, serving as nanoparticle guiding ligands. Their dedication promises innovative contributions to our ongoing research.
Rasmus Enno BSc: Rasmus, our MSc student, has delved into the fall semester with a focus on the expression of recombinant receptors for homing peptides. His work also involves the development of novel methodologies for the identification of homing peptide receptors, showcasing a commitment to advancing our understanding in this field.
Kristina Rabi: As a BSc student, Kristina has joined our laboratory with a dual role. Beyond her research efforts aimed at improving in vivo playoff technology, she actively supports our team as a lab assistant, bringing valuable contributions to our phage display-related activities.
As we welcome these talented individuals, we look forward to the diverse perspectives and collaborative spirit they bring to our team. Together, we aim to make significant strides in the fascinating realms of nanomedicine and precision targeting.
Nanomedicine Discussions and Collaborative Visit in Colorado
From May 4 to 6, 2023, Professor Teesalu participated in the "Mechanisms and Barriers in Nanomedicine" conference in Golden, Colorado. The conference, organized by fellow Ruoslahti lab alumnus Professor Dmitri Simberg, provided a platform for Prof. Teesalu to share insights through an oral presentation. For more information, you can visit the conference website: Conference Link.
After the conference, Prof. Teesalu visited Professor Gregory Robertson's lab at Colorado State University (Robertson Lab). During the visit, he gave a seminar and engaged in discussions about collaborative work, particularly on targeting mycobacterial infections. This research is funded by the Gates Foundation.
Farewell to Assintant Professor Lorena Simón Gracia: A Decade of Dedication and a New Chapter
After more than a decade of invaluable contributions, Lorena has made the decision to embark on a new chapter in her career back in her native Spain. As she transitions into this exciting phase, we extend our heartfelt wishes for success both within and outside the laboratory.
Although Lorena is leaving our day-to-day team, she will remain connected to the Laboratory of Precision and Nanomedicine as a visiting researcher. This continuity in collaboration reflects the enduring bonds forged over the years and the ongoing exchange of knowledge and ideas.
To commemorate Lorena's time with us, we organized a special canoeing day trip on the picturesque Ahja river. As Lorena sets sail for new horizons, we express our deepest gratitude for her dedication and hard work. We look forward to the continued collaboration in her new role and wish her the very best in all her future endeavors, both personally and professionally. ¡Buena suerte, Lorena!
Congratulations to Anni Lepland and Kristina Kiisholts for defending their PhD theses!
We extend our heartfelt congratulations to Anni Lepland and Kristina Kiisholts for their successful defense of their PhD theses, showcasing significant contributions to the field of precision nanomedicine.
Kristina Kiisholts defended her thesis, titled "Peptide-based drug carriers and preclinical nanomedicine applications for endometriosis treatment," on April 28, 2023. This collaborative and interdisciplinary study, cosupervised by Professors Kaido Kurrikoff, Andres Salumets, Tambet Teesalu, and Ülo Langel, explored peptide-based nanomedical applications for endometriosis treatment. The opponent for the defense was Professor Pirjo Laakkonen from the University of Helsinki (Finland). The findings from Kiisholts' thesis propose promising peptide-based approaches for endometriosis treatment with potential translational relevance. Link to more details and Link to Thesis.
Anni Lepland's thesis, "Precision targeting of tumor-associated macrophages in triple negative breast cancer," focused on peptide-guided targeting of tumor macrophages in breast cancer. Supervised by Professors Pablo D Scodeller and Tambet Teesalu, the thesis defense took place with Assistant Professor Maija Hollmén from the University of Turku (Finland) as the opponent. Notably, Lepland's thesis was honored with the Best Doctoral Thesis Award for 2023 in Biomedicine in December 2023. Link to Thesis and More details on the award.
We wish both Anni Lepland and Kristina Kiisholts continued success in their future careers, confident that their research will leave a lasting impact on the field of nanomedicine.
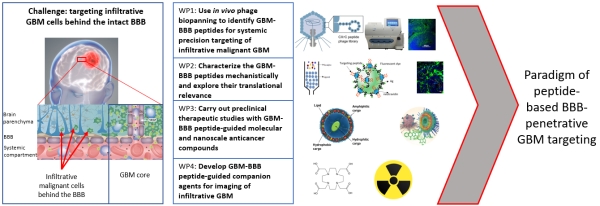
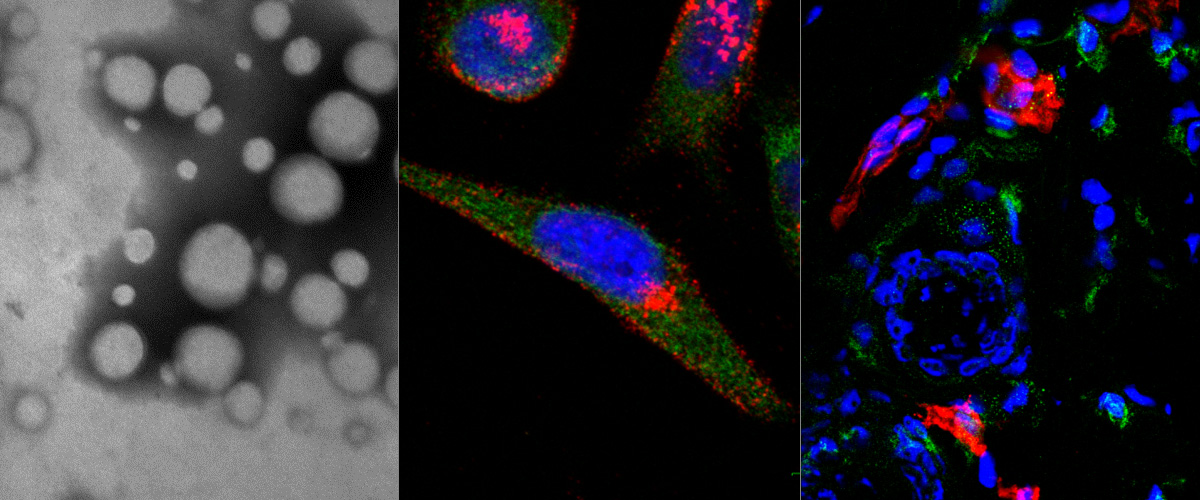
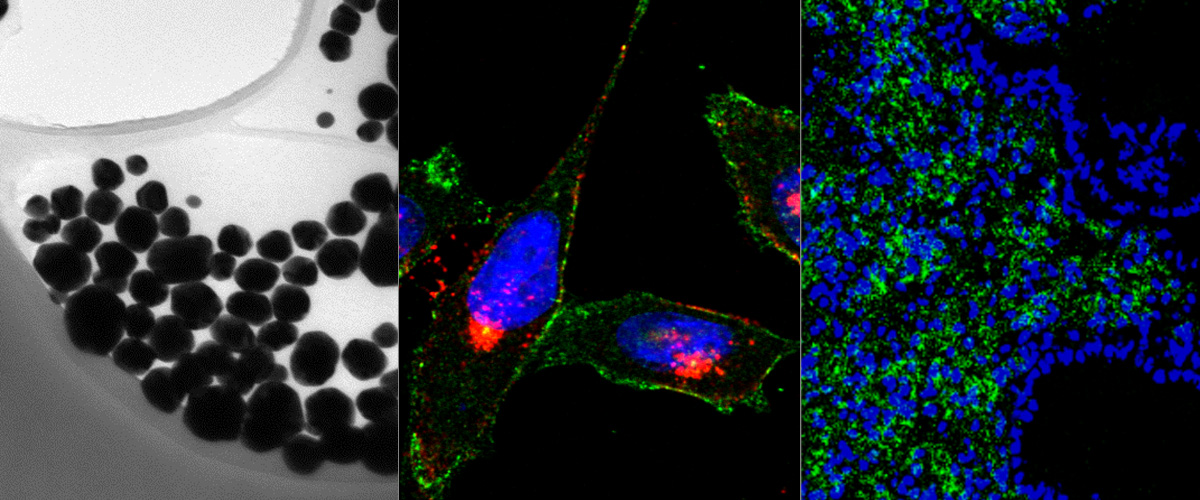
Success with our Estonian Research Council project "Precision targeting of infiltrative glioblastoma"
We are delighted to announce positive funding decision by the Estonian Research Council on our 5-year research project "Precision targeting of infiltrative glioblastoma". The project proposes studies on identification and preclinical validation of homing peptides able to penetrate the blood brain barrier and to target infiltrative glioblastoma cells. The project is expected to lead to identification and mechanistic characterization of the infiltrative glioblastoma targeting peptides and execution of the preclinical studies with peptide-guided therapeutic nanoparticles and radiotracers. These studies may lead to a new paradigm of peptide-based precision management of infiltrative glioblastoma.
Prof. Erkki Ruoslahti conferred the degree of Honorary Doctor of Biomedicine of UniTartu
Disntinguished professor Erkki Ruoslahti, a longtime mentor and present collaborator of Prof. Teesalu, has been conferred the degree of Honorary Doctor of Biomedicine of the University of Tartu for "cooperation with the University of Tartu in developing the research fields of cancer biology, nanomedicine and drug development".
Prof. Ruoslahti is a member of the US National Academy of Sciences since 1999 and has received several prestigious awards (e.g. Canada Gairdner International Award in 1997, Japan Prize in Cell Biology in 2005, and Albert Lasker Award for Basic Medical Research in 2022).