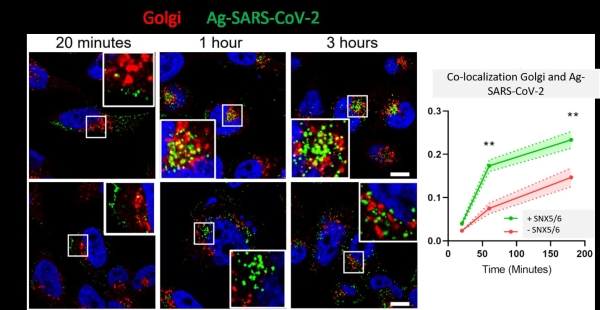
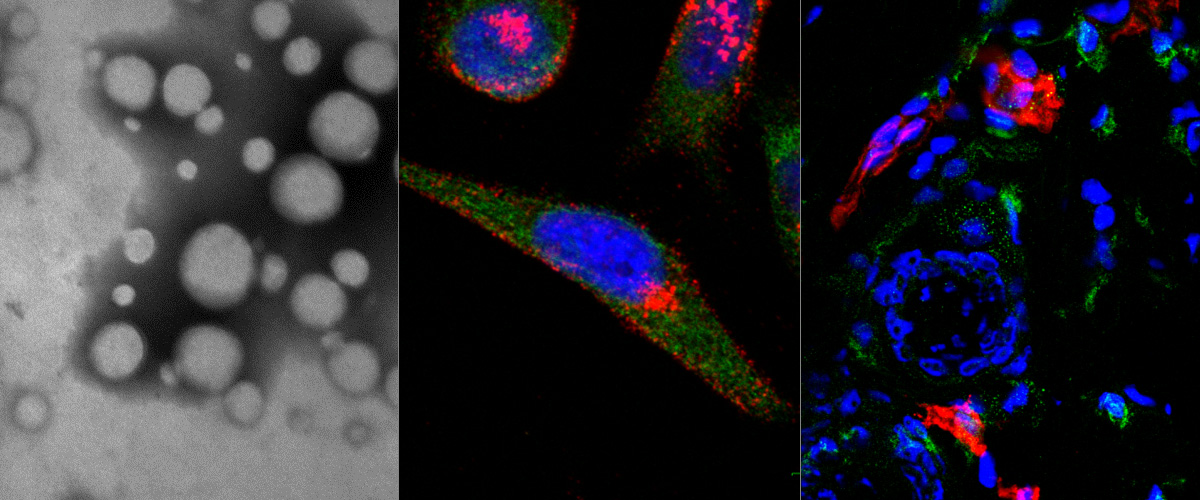
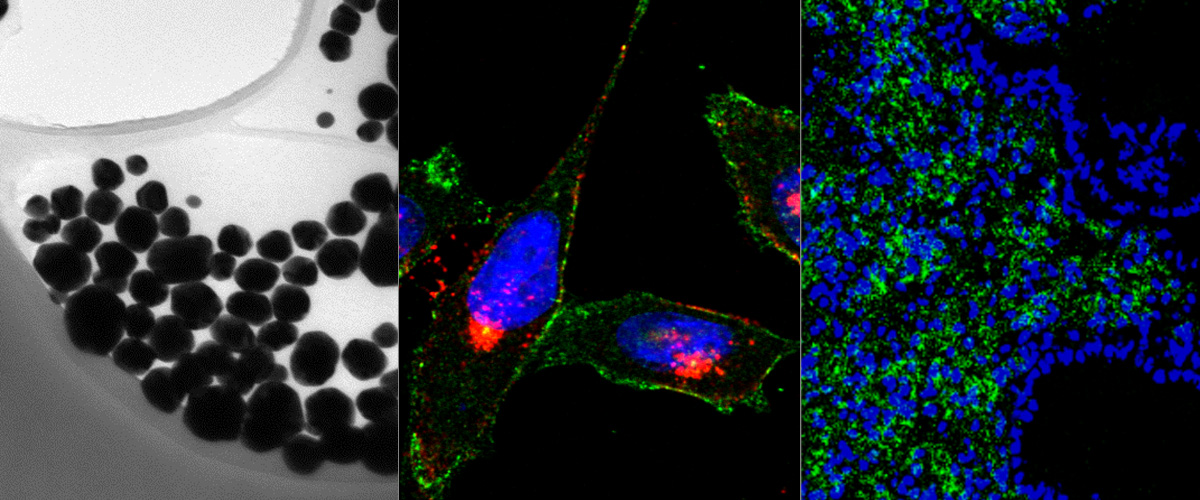
Our recent publication in the journal Proceedings of the National Academy of Sciences (PNAS) explains how the SARS-CoV-2 coronavirus can hijack an intracellular pathway to increase its infectivity. In collaboration with researchers at the University of Bristol (Dr. Boris Simonetti, Dr. Peter Cullen, Dr. Yohei Yamauchi), we studied how the SARS-CoV-2 and other viruses use the host intracellular machinery to enter into the cells. We previously showed that neuropilin-1 (NRP-1) is a co-receptor of the SARS-CoV-2 virus and that the binding to NRP-1 through the specific CendR motif displayed on the spike protein facilitates the cellular entry an infectivity of the virus. In this new study, we show how the NRP-1 and its ligands (nanoparticles displaying the CendR motif) are intracellularly transported by the ESCPE-1 protein complex. Knocking down essential proteins of the ESCPE-1 complex significantly decreased the internalization of the nanoparticles and, importantly, the infectivity of the corona virus. These findings could serve to develop new therapeutics that can interfere with the ESCPE-1 complex and prevent the NRP-1-mediated infection of the SARS-CoV-2 virus and other viruses.
Media:
https://www.sciencedaily.com/releases/2022/06/220614095546.htm