https://pubmed.ncbi.nlm.nih.gov/33001014
Abstract
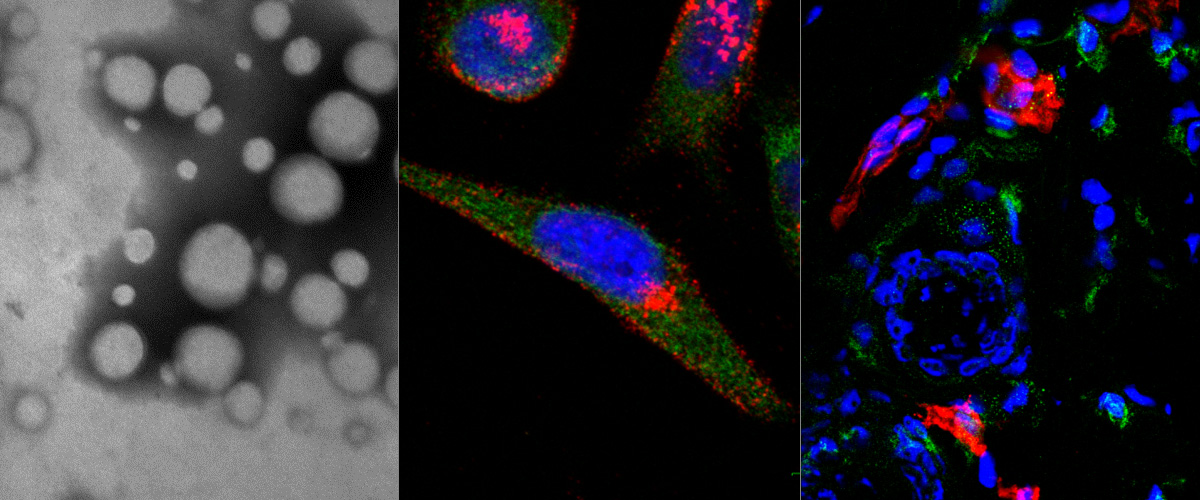
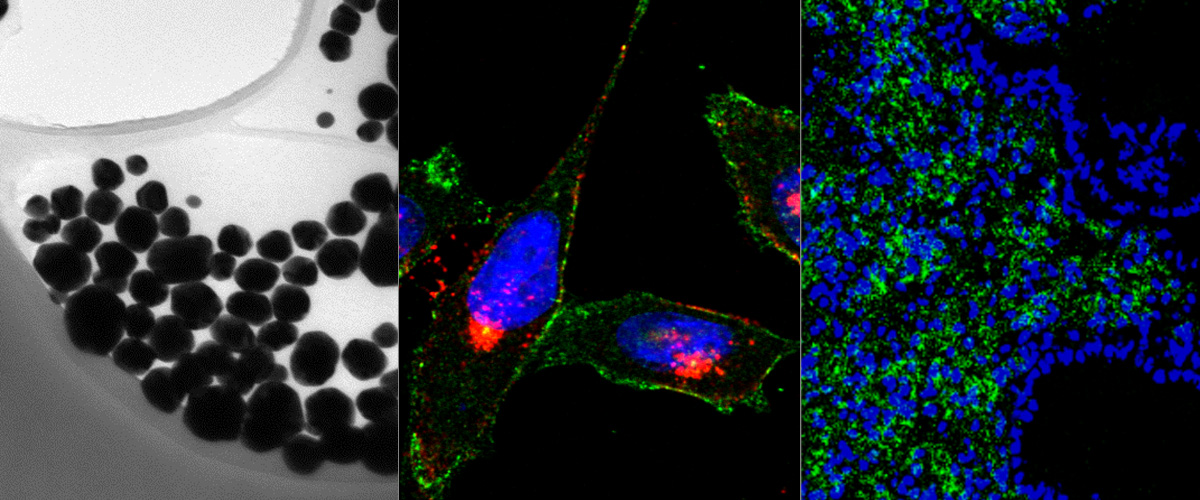
Background: Conjugation to clinical-grade tumor penetrating iRGD peptide is a widely used strategy to improve tumor homing, extravasation, and penetration of cancer drugs and tumor imaging agents. The C domain of extracellular matrix molecule Tenascin-C (TNC-C) is upregulated in solid tumors and represents an attractive target for clinical-grade single-chain antibody-based vehicles for tumor delivery drugs and imaging agents. Objective: To study the effect of C-terminal genetic fusion of the iRGD peptide to recombinant anti-TNC-C single-chain antibody clone G11 on systemic tumor homing and extravasation. Methods: Enzyme-linked immunosorbent assay was used to study the interaction of parental and iRGD-fused anti-TNC-C single-chain antibodies with C domain of tenascin-C and αVβ3 integrins. For systemic homing studies, fluorescein-labeled ScFV G11-iRGD and ScFV G11 antibodies were administered in U87-MG glioblastoma xenograft mice, and their biodistribution was studied by confocal imaging of tissue sections stained with markers of blood vessels and Tenascin C immunoreactivity. Results: In a cell-free system, iRGD fusion to ScFV G11 conferred the antibody a robust ability to bind αVβ3 integrins. The fluorescein labeling of ScFV G11-iRGD did not affect its target binding activity. In U87-MG mice, iRGD fusion to ScFV G11 antibodies improved their homing to tumor blood vessels, extravasation, and penetration of tumor parenchyma. Conclusion: The genetic fusion of iRGD tumor penetrating peptide to non-internalizing affinity targeting ligands may improve their tumor tropism and parenchymal penetration for more efficient delivery of imaging and therapeutic agents into solid tumor lesions.