https://doi.org/10.1002/adtp.202000097
Abstract
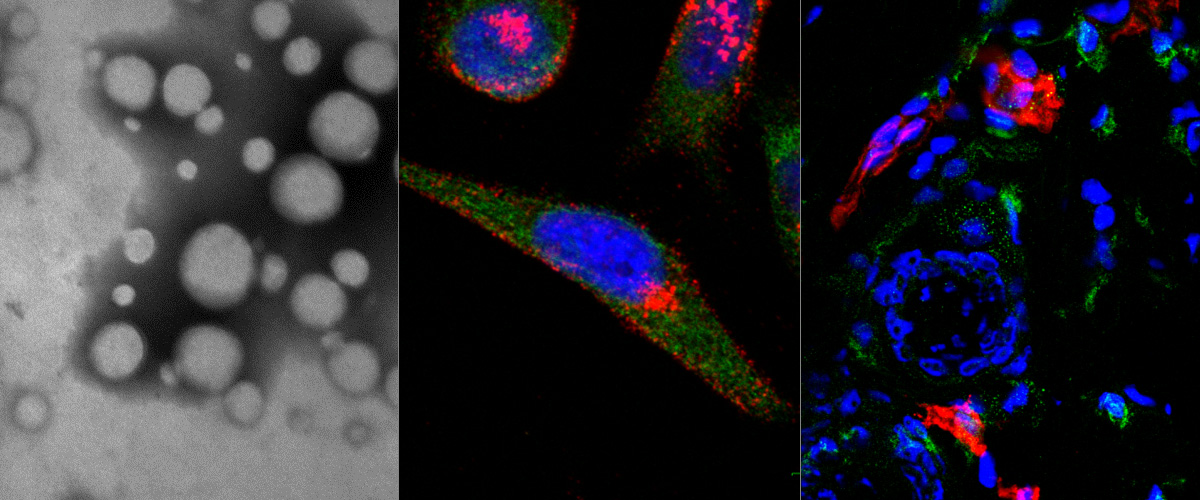
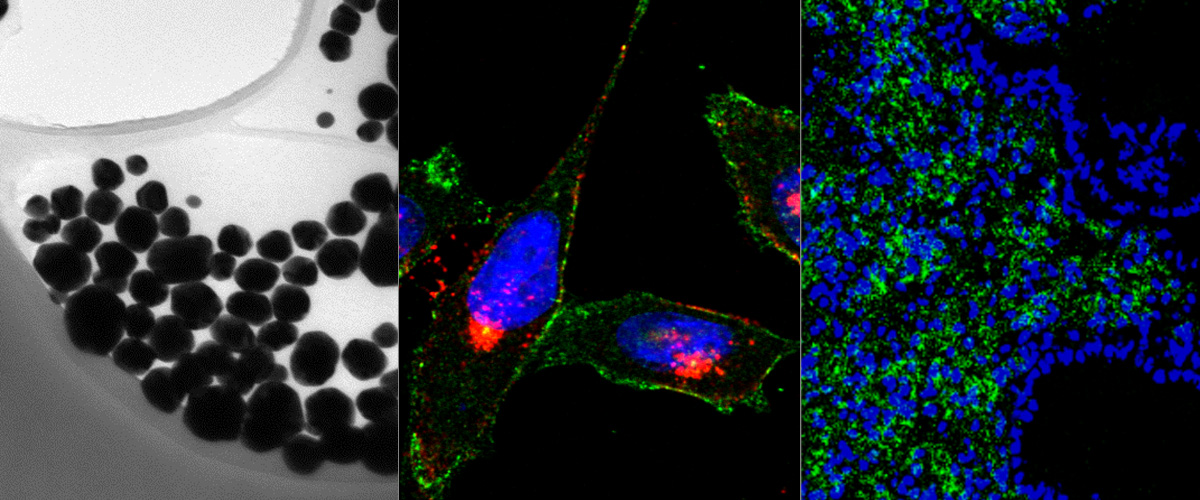
Silver nanoparticles (AgNP) can be tracked in cells and tissues by optical imaging and isotopic fingerprinting. AgNPs are particularly useful as endocytosis probes as they can be rapidly dissolved with a biocompatible etching solution to eliminate extracellular NPs.Here, affinity‐targeted AgNPs are evaluated as therapeutic carriers of a potent cytotoxic compound, monomethyl auristatin E (MMAE). AgNPs are coated with MMAE via a lysosomal protease cathepsin B sensitive linker and functionalized with a prototypic CendR peptide (RPARPAR) that targets neuropilin‐1 (NRP‐1). This gives the AgNPs dual tumor specificity as both cathepsin B and NRP‐1 are overexpressed in many types of solid tumors. Cellular imaging, flow cytometry, viability assays, and high‐performance liquid chromatography‐mass spectrometry (HPLC‐MS) analysis show that the RPARPAR–MMAE–AgNPs are internalized and induce apoptotic cell death in NRP‐1‐positive PPC‐1 prostate cancer cells while sparing NRP‐1‐negative M21 melanoma cells. Furthermore, in a mixed culture of PPC‐1 and M21 cells, RPARPAR–MMAE–AgNP treatment selectively eliminates NRP‐1‐positive PPC‐1 cells. The study demonstrates that affinity‐targeted AgNPs can be used as carriers to selectively deliver and potentiate the activity of cytotoxic compounds in vitro, and that treatment with a biocompatible etching solution can be used to control the internalization and, thus, the cytotoxicity of these AgNPs.