https://www.ncbi.nlm.nih.gov/pubmed/28468593
Abstract
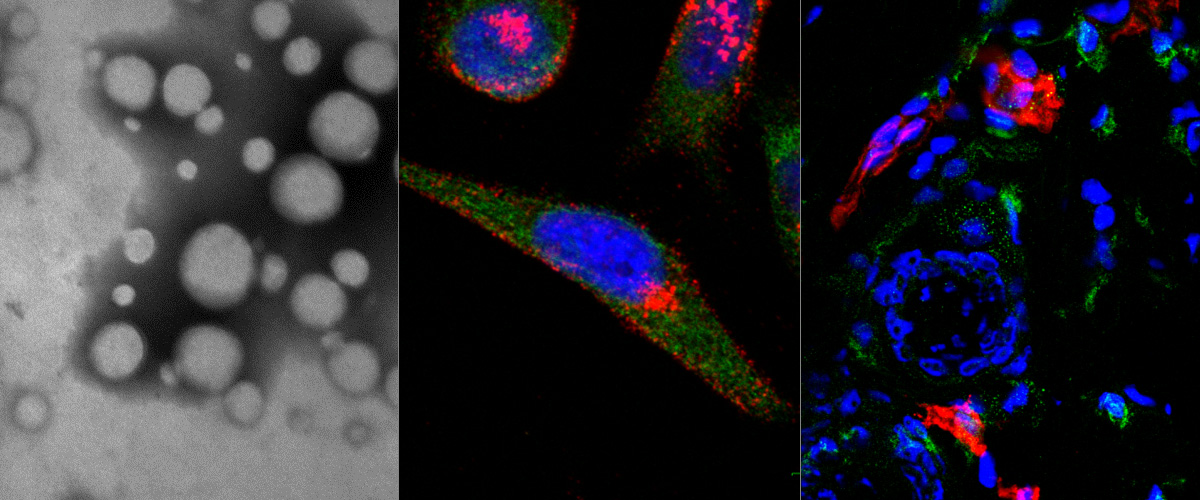
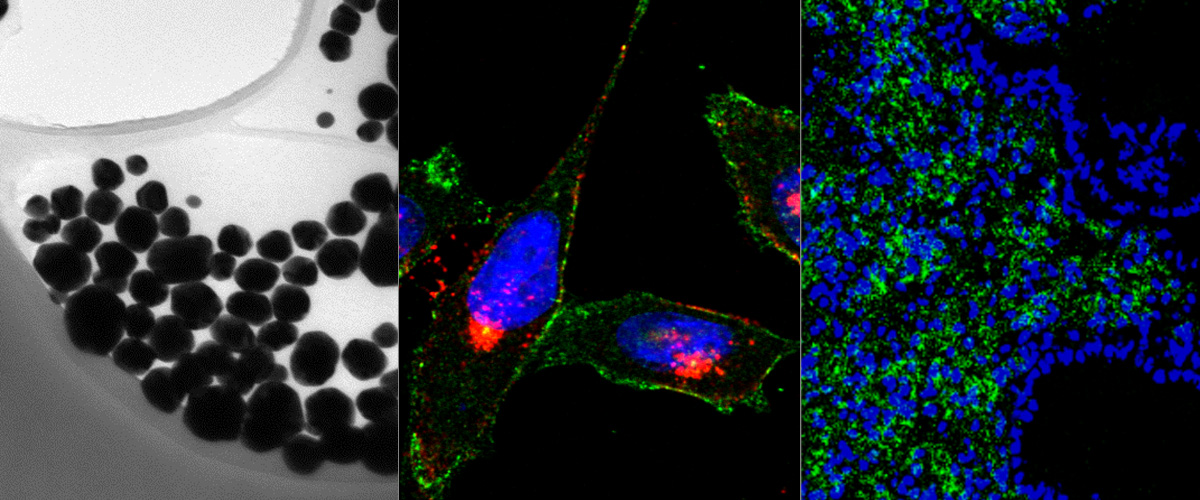
Peritoneal carcinomatosis results from dissemination of solid tumors in the peritoneal cavity, and is a common site of metastasis in patients with carcinomas of gastrointestinal or gynecological origin. Peritoneal carcinomatosis treatment is challenging as poorly vascularized, disseminated peritoneal micro-tumors are shielded from systemic anticancer drugs and drive tumor regrowth. Here, we describe the identification and validation of a tumor homing peptide CKRDLSRRC (IP3), which upon intraperitoneal administration delivers payloads to peritoneal metastases. IP3 peptide was identified by in vivo phage display on a mouse model of peritoneal carcinomatosis of gastric origin (MKN-45P), using high-throughput sequencing of the peptide-encoding region of phage genome as a readout. The IP3 peptide contains a hyaluronan-binding motif, and fluorescein-labeled IP3 peptide bound to immobilized hyaluronan in vitro. After intraperitoneal administration in mice bearing peritoneal metastases of gastric and colon origin, IP3 peptide homed robustly to macrophage-rich regions in peritoneal tumors, including poorly vascularized micro-tumors. Finally, we show that IP3 functionalization conferred silver nanoparticles the ability to home to peritoneal tumors of gastric and colonic origin, suggesting that it could facilitate targeted delivery of nanoscale payloads to peritoneal tumors. Collectively, our study suggests that the IP3 peptide has potential applications for targeting drugs, nanoparticles, and imaging agents to peritoneal tumors.